YHEC has a proven track record of creating all of the materials for a health technology assessment (HTA) submission, as well as preparing the submission document itself. As such, we can seamlessly integrate the following into the submission dossier:
- Systematic literature reviews
- Evidence synthesis (mixed treatment comparison, conventional meta-analysis)
- Burden of illness reviews
- Economic modelling (cost-effectiveness and budget impact)
- Patient reported outcomes
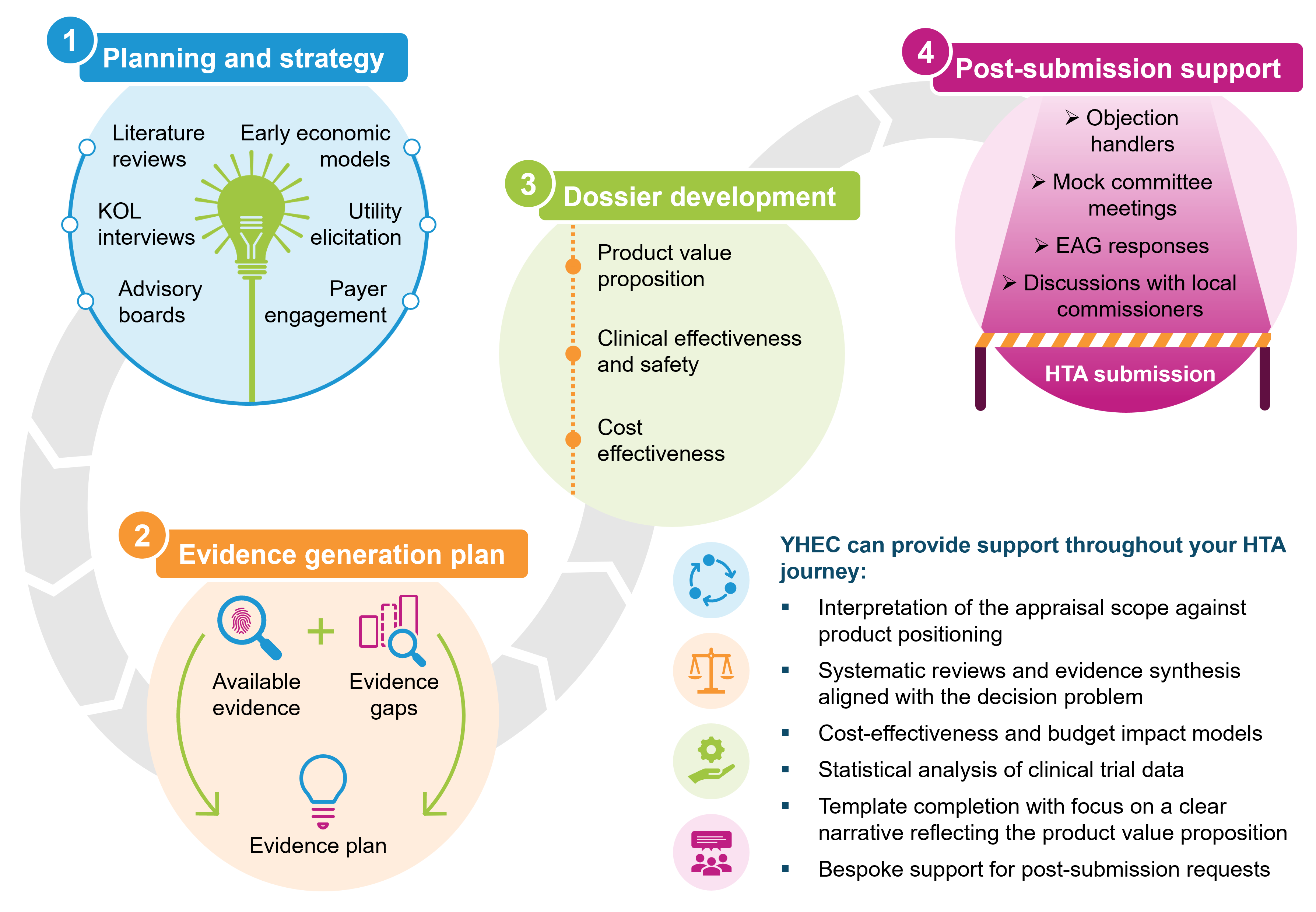 YHEC regularly prepares dossiers for submission to the National Institute for Health and Care Excellence (NICE) in whichever programme a medical device is routed to. We have vast experience of submissions to both the Medical Technologies Evaluation Programme (MTEP) and the Technology appraisal (TA) programme and have also supported submissions to the Diagnostic Appraisal Programme (DAP). YHEC also has experience of working on submissions to other major international agencies.
YHEC regularly prepares dossiers for submission to the National Institute for Health and Care Excellence (NICE) in whichever programme a medical device is routed to. We have vast experience of submissions to both the Medical Technologies Evaluation Programme (MTEP) and the Technology appraisal (TA) programme and have also supported submissions to the Diagnostic Appraisal Programme (DAP). YHEC also has experience of working on submissions to other major international agencies.
YHEC will support you from beginning to end with your dossier preparation covering:
- Preparation of an outline including key messaging for each section
- Creation of content for all sections of the dossier
- Section specific, as well as whole document review in order to streamline the review process and have key people critique key sections of the document at the right time
- Overall editorial control
- Highlighting text that is commercial in confidence or academic in confidence
- Preparation of reference packs
- Rapid post-submission support and reanalyses if needed
- Organisation of mock appraisal committee meetings
- Attendance at committee meetings
Examples of recent HTA submissions include:
- NICE submission for treating liver cancer
- NICE MTEP submission in the area of colorectal cancer (diagnostic)
- NICE MTEP submission in the area of infection prevention
- NICE STA submission in the area of implantable devices

Recent Comments