Please click on the headings below for further information.
Systematic Review
Systematic Review
We can help you to:
- Define your research question
- Develop a review protocol to ensure that the review will help you to answer those questions.
- Undertake extensive literature searches to ensure all studies relevant to the review question and the NMA are identified
- Assess the risk of bias of the individual trials
- Extract the relevant data required to assess the feasibility of a NMA and to inform any subsequent statistical analyses
Feasibility Assessment
Feasibility Assessment
A key assumption when conducting a NMA is that the trials in the network do not differ in any characteristics that may impact the treatment effect.
We consider the similarity of key characteristics of the trials in relation to:
- Trial design/methodology
- Patient characteristics at baseline
- Treatments received
- Outcomes reported
We also consider the availability of outcome data for each outcome of interest in each of the trials. Based on the data available, we can then determine whether a connected network can be made for each outcome of interest.
NMA
NMA
We can conduct network meta-analyses, through our partnership with Quantics, to compare the efficacy, safety or tolerability of two or more treatments. We can help with simple NMA as well as more complex network meta-regressions and we can advise you on the best methods to use for the data available.
Critiquing an NMA
Critiquing an NMA
We have extensive experience of assessing the quality of both peer-reviewed publications and health technology assessment submissions. In conjunction with Quantics, we can appraise all aspects of systematic reviews and NMA.
The quality of published NMAs is highly variable. Quantics and YHEC can help you to answer questions such as:
- Are the search terms and search resources appropriate to ensure that as many relevant trials as possible will have been identified?
- Have the data been correctly extracted from the publications?
- Has the similarity of the trials been considered before combining them in a network?
- Have the most appropriate statistical methods been used to conduct the NMA?
- Are the published results reproducible?
- Have the results been correctly interpreted?
What is a Network Meta-analysis?
Network meta-analysis (NMA) is an umbrella term which can be used to describe indirect and mixed treatment comparisons (ITC/MTC). They are statistical tools for assessing, for example, the comparative efficacy and safety of interventions when:
- There is no direct evidence between the interventions of interest
- There is insufficient direct evidence or
- There are more than two interventions that need to be compared
NMA methods are developing rapidly and are already being widely used in health care to inform decisions which require an understanding of the comparative effectiveness of different interventions.
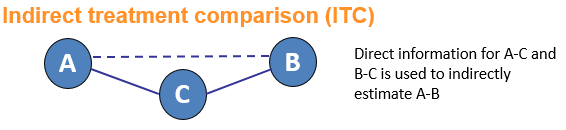
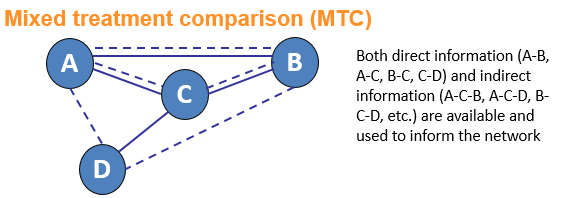
Why conduct an NMA?
HTA submission
- NMAs are now accepted as part of health technology assessments (HTA) in many countries including England [the National Institute for Health and Care Excellence (NICE)], France (Haute Autorité de Santé), Canada (Canadian Agency for Drugs and Technologies in Health) and Australia (Pharmaceutical Benefits Advisory Committee)
- We can provide all of the documentation required by the specific HTA agency and can help you to populate the relevant section of the submission template
Inform an economic model
- At YHEC, we have experience of building and adapting economic models for hundreds of health care technologies. The outputs of NMA can be used to inform the model inputs
Produce a publication
- We can support you to carry out a systematic review and conduct NMA in line with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) NMA reporting guidelines
Inform future trials
- We can help you interpret the results of your trial within the context of comparator trials or, inform plans for future clinical trials by assessing where your trial could fit in the evidence network.
How can we help you?
As with a standard pairwise meta-analysis, the validity of an NMA depends on:
- The adequacy of the evidence base
- The similarity of the trials
These issues underpin the focus of the systematic review and feasibility assessment of whether an NMA is possible. Formal feasibility assessment of the similarity of trials is crucial to a high quality and robust network.
We can work with you right through from question development to conducting the NMA or we can review and critique existing or published NMA.
 Local Health and Public Sector Organisations
Local Health and Public Sector Organisations